#New method for the development of choline acetyltransferase inhibitors

“#New method for the development of choline acetyltransferase inhibitors”
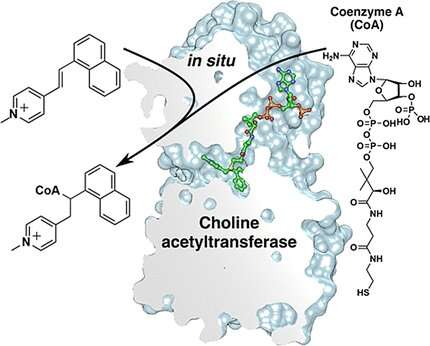
The enzyme choline acetyltransferase (ChAT) catalyzes the synthesis of the neurotransmitter acetylcholine and could be a target molecule for pharmaceuticals. A Swedish research team has now determined the mechanism by which arylvinylpyridinium (AVP), a known class of ChAT inhibitors, functions. As they report in the journal Angewandte Chemie, ChAT manufactures the actual agent itself by attaching coenzyme A (CoA) to AVP.
Acetylcholine passes on nerve impulses in synapses, among other things. Changes in the amount or activity of ChAT, which produces acetylcholine from choline and acetylcoenzyme A (AcCoA), have been observed in a variety of diseases, such as Alzheimer’s disease, schizophrenia, congenital disruption of signal conduction between nerves and muscles, and chronic viral infections. In addition, inhibition of ChAT could serve as an effective treatment for poisoning by organophosphate neurotoxins.
To date, suitable ChAT inhibitors have been unavailable. Arylvinylpyridinium compounds (AVPs) were good inhibitors in vitro, but their pharmacological profile proved to be inconsistent. A team at the Swedish Defense Research Agency and Umeå University (Sweden) led by Fredrik Ekström has now been able to clarify the inhibition mechanism of AVPs and provide insights that could be used as the basis for developing ChAT inhibitors with better effectiveness and bioactivity.
The researchers were able to demonstrate that the actual bioactive agents are not the AVPs themselves, but the adduct they form with CoA. Connection of these two components is carried out by ChAT itself. The enzyme builds its own inhibitor starting from an exogenic precursor (AVP) and an endogenic co-substrate (CoA). The reaction is an unusual hydrothiolation, which forms a bond between the thiol group of the CoA and the vinyl group of the AVP.
The binding and catalytic domains in ChAT form a narrow ‘tunnel’ that passes across the enzyme. X-ray crystallographic studies of human ChAT in the presence of CoA and AVP showed that the inhibiting adduct is embedded deep within this tunnel. Interactions with hydrophobic pockets near the choline binding site have significant effects on the strength of ChAT inhibitors.
The researchers hope that their discoveries will allow for the development of novel ChAT inhibitors with improved strength and duration of activity.
Daniel Wiktelius et al. In Situ Assembly of Choline Acetyltransferase Ligands by a Hydrothiolation Reaction Reveals Key Determinants for Inhibitor Design, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202011989
Citation:
New method for the development of choline acetyltransferase inhibitors (2020, December 21)
retrieved 21 December 2020
from https://phys.org/news/2020-12-method-choline-acetyltransferase-inhibitors.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.



