#X-ray study explores potential of hepatitis C drugs to treat COVID-19

“#X-ray study explores potential of hepatitis C drugs to treat COVID-19”
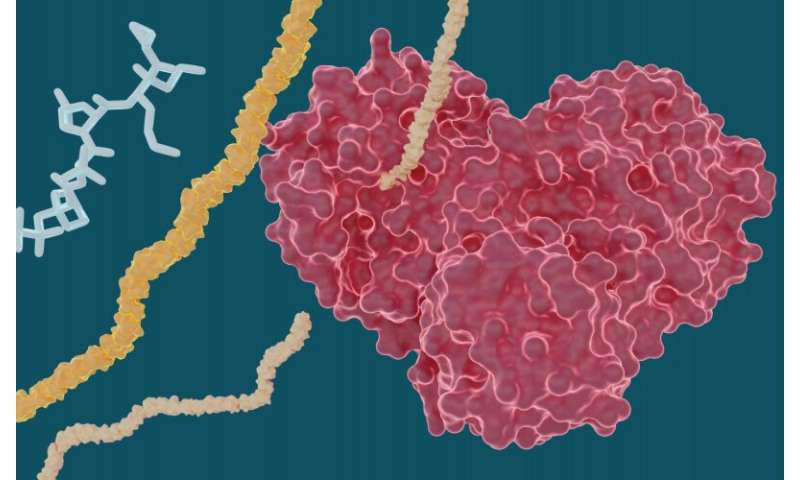
Experiments led by researchers at the Department of Energy’s Oak Ridge National Laboratory have determined that several hepatitis C drugs can inhibit the SARS-CoV-2 main protease, a crucial protein enzyme that enables the novel coronavirus to reproduce.
Inhibiting, or blocking, this protease from functioning is vital to stopping the virus from spreading in patients with COVID-19. The study, published in the journal Structure, is part of efforts to quickly develop pharmaceutical treatments for COVID-19 by repurposing existing drugs known to effectively treat other viral diseases.
“Currently, there are no inhibitors approved by the Food and Drug Administration that target the SARS-CoV-2 main protease,” said ORNL lead author Daniel Kneller. “What we found is that hepatitis C drugs bind to and inhibit the coronavirus protease. This is an important first step in determining whether these drugs should be considered as potential repurposing candidates to treat COVID-19.”
The SARS-CoV-2 coronavirus spreads by expressing long chains of polyproteins that must be cut by the main protease to become functional proteins, making the protease an important drug target for researchers and drug developers.
In the study, the team looked at several well-known drug molecules for potential repurposing efforts including leupeptin, a naturally occurring protease inhibitor, and three FDA-approved hepatitis C protease inhibitors: telaprevir, narlaprevir and boceprevir.
The team performed room temperature X-ray measurements to build a three-dimensional map that revealed how the atoms were arranged and where chemical bonds formed between the protease and the drug inhibitor molecules.
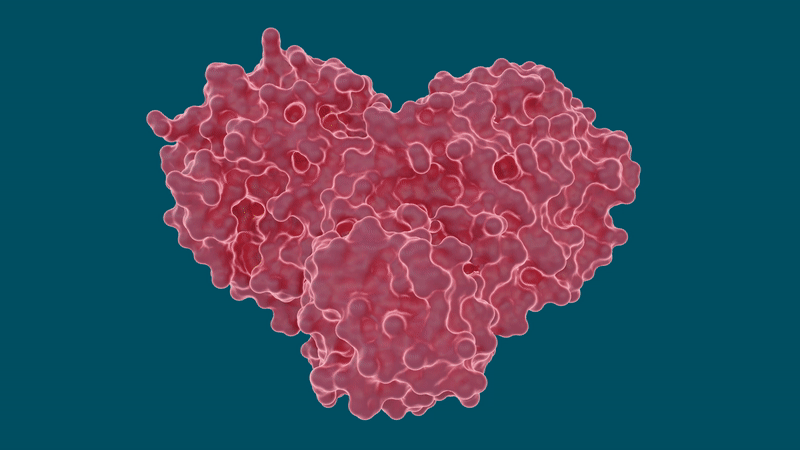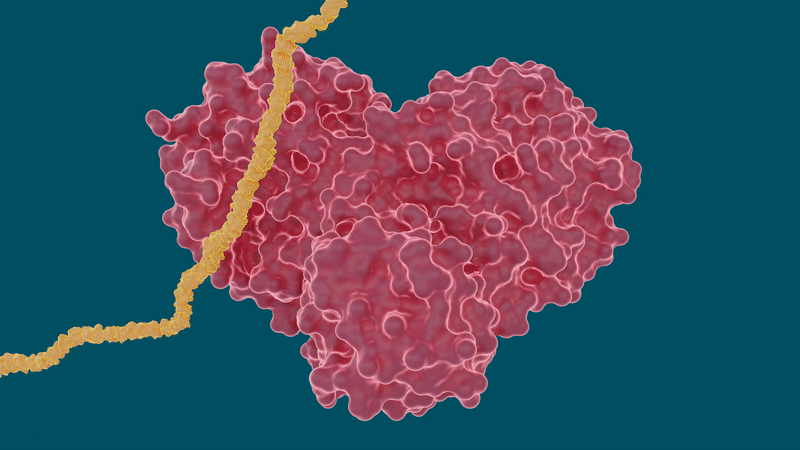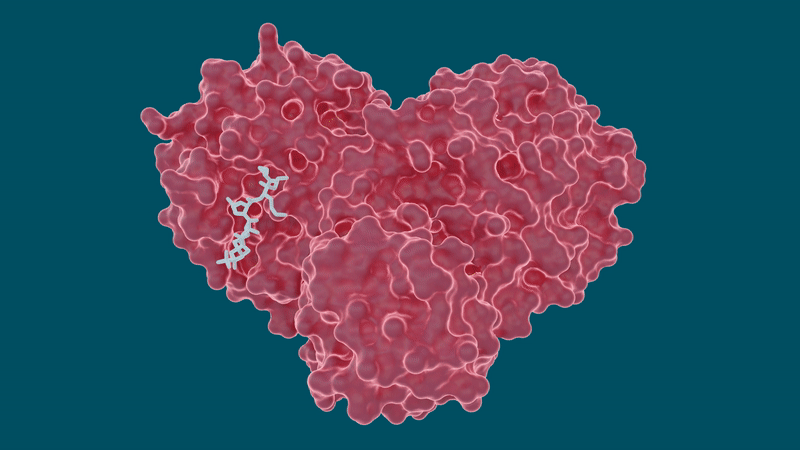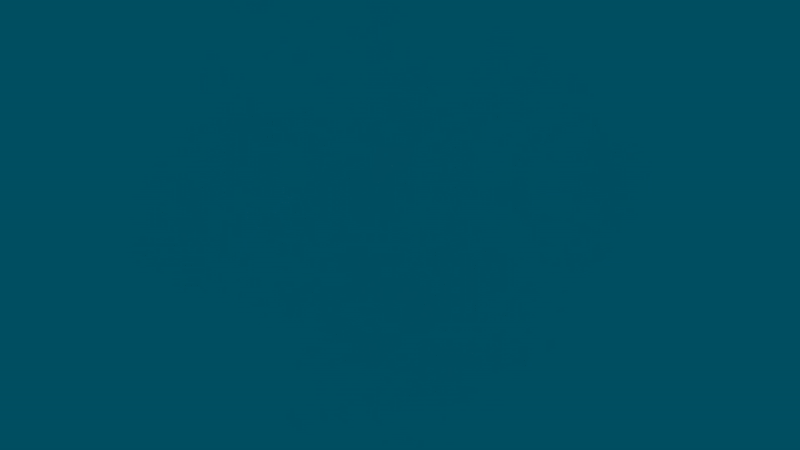
The experiments yielded promising results for certain hepatitis C drugs in their ability to bind and inhibit the SARS-CoV-2 main protease—particularly boceprevir and narlaprevir. Leupeptin exhibited a low binding affinity and was ruled out as a viable candidate.
To better understand how well or how tightly the inhibitors bind to the protease, they used in vitro enzyme kinetics, a technique that enables researchers to study the protease and the inhibitor in a test tube to measure the inhibitor’s binding affinity, or compatibility, with the protease. The higher the binding affinity, the more effective the inhibitor is at blocking the protease from functioning.
“What we’re doing is laying the molecular foundation for these potential drug repurposing inhibitors by revealing their mode of action,” said ORNL corresponding author Andrey Kovalevsky. “We show on a molecular level how they bind, where they bind, and what they’re doing to the enzyme shape. And, with in vitro kinetics, we also know how well they bind. Each piece of information gets us one step closer to realizing how to stop the virus.”
The study also sheds light on a peculiar behavior of the protease’s ability to change or adapt its shape according to the size and structure of the inhibitor molecule it binds to. Pockets within the protease where a drug molecule would attach are highly malleable, or flexible, and can either open or close to an extent depending on the size of the drug molecules.
Before the paper was published, the researchers made their data publicly available to inform and assist the scientific and medical communities. More research, including clinical trials, is necessary to validate the drugs’ efficacy and safety as a COVID-19 treatment.
“The research suggests that hepatitis C inhibitors are worth thinking about as potential repurposing candidates. Immediately releasing our data allows the scientific community to start looking at the interactions between these inhibitors and the protease,” said ORNL corresponding author Leighton Coates. “You can’t design a drug without knowing how it works on a molecular level, and the data we’re providing is exactly what developers need to design stronger, more tightly binding drugs for more effective treatments.”
Daniel W. Kneller et al, Malleability of the SARS-CoV-2 3CL Mpro Active-Site Cavity Facilitates Binding of Clinical Antivirals, Structure (2020). DOI: 10.1016/j.str.2020.10.007
Citation:
X-ray study explores potential of hepatitis C drugs to treat COVID-19 (2020, November 16)
retrieved 17 November 2020
from https://medicalxpress.com/news/2020-11-x-ray-explores-potential-hepatitis-drugs.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.


