#Using ‘counterfactuals’ to verify predictions of drug safety

Table of Contents
“Using ‘counterfactuals’ to verify predictions of drug safety”
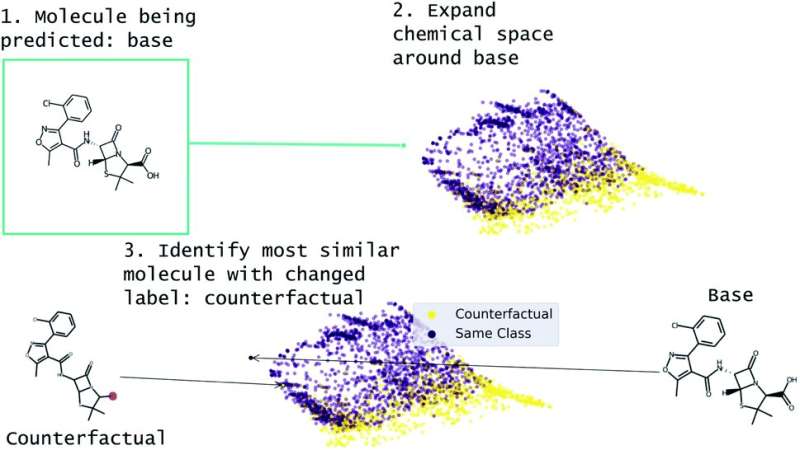
Scientists rely increasingly on models trained with machine learning to provide solutions to complex problems. But how do we know the solutions are trustworthy when the complex algorithms the models use are not easily interrogated or able to explain their decisions to humans?
That trust is especially crucial in drug discovery, for example, where machine learning is used to sort through millions of potentially toxic compounds to determine which might be safe candidates for pharmaceutical drugs.
“There have been some high-profile accidents in computer science where a model could predict things quite well, but the predictions weren’t based on anything meaningful,” says Andrew White associate professor of chemical engineering at the University of Rochester, in an interview with Chemistry World.
White and his lab have developed a new “counterfactual” method, described in Chemical Science, that can be used with any molecular structure-based machine learning model to better understand how the model arrived at a conclusion.
Counterfactuals can tell researchers “the smallest change to the features that would alter the prediction,” says lead author Geemi Wellawatte, a Ph.D. student in White’s lab. “In other words, a counterfactual is an example as close to the original, but with a different outcome.”
Counterfactuals can help researchers quickly pinpoint why a model made a prediction, and whether it is valid.
The paper identifies three examples of how the new method, called MMACE (Molecular Model Agonistic Counterfactual Explanations), can be used to explain why:
- a molecule is predicted to permeate the blood-brain barrier
- a small molecule is predicted to be soluble
- a molecule is predicted to inhibit HIVs
The lab had to overcome some major challenges in developing MMACE. They needed a method that could be adapted for the wide array of machine-learning methods that are used in chemistry. In addition, searching for the most-similar molecule for any given scenario was also challenging because of the sheer number of possible candidate molecules.

Coauthor Aditi Seshadri in White’s lab helped solve that problem by suggesting the group adapt the STONED (Superfast traversal, optimization, novelty, exploration, and discovery) algorithm developed at the University of Toronto. STONED efficiently generates similar molecules, the fuel for counterfactual generation. Seshadri is an undergraduate researcher in White’s lab and was able to help on the project via a Rochester summer research program called “Discover.”
White says his team is continuing to improve MMACE, by trying other databases in their search for most similar molecules, for example, and refining the definition of molecular similarity.
Geemi P. Wellawatte et al, Model agnostic generation of counterfactual explanations for molecules, Chemical Science (2022). DOI: 10.1039/D1SC05259D
Citation:
Using ‘counterfactuals’ to verify predictions of drug safety (2022, May 2)
retrieved 2 May 2022
from https://phys.org/news/2022-05-counterfactuals-drug-safety.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.



