#Unleashing disordered rocksalt oxides as cathodes for rechargeable magnesium batteries

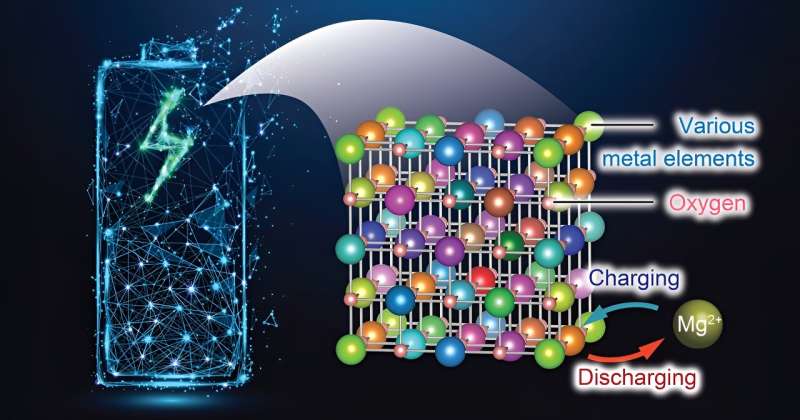
Researchers at Tohoku University have made a advancement in battery technology by developing a novel cathode material for rechargeable magnesium batteries (RMBs) that enables efficient charging and discharging even at low temperatures. This innovative material, leveraging an enhanced rock-salt structure, promises to usher in a new era of energy storage solutions that are more affordable, safer, and higher in capacity.
Details of the findings were published in the Journal of Materials Chemistry A.
The study showcases a considerable improvement in magnesium (Mg) diffusion within a rock-salt structure, a critical advancement since the denseness of atoms in this configuration had previously impeded Mg migration. By introducing a strategic mixture of seven different metallic elements, the research team created a crystal structure abundant in stable cation vacancies, facilitating easier Mg insertion and extraction.
This represents the first utilization of rocksalt oxide as a cathode material for RMBs. The high-entropy strategy employed by the researchers allowed the cation defects to activate the rocksalt oxide cathode.
The development also addresses a key limitation of RMBs—the difficulty of Mg transport within solid materials. Until now, high temperatures were necessary to enhance Mg mobility in conventional cathode materials, such as those with a spinel structure. However, the material unveiled by Tohoku University researchers operates efficiently at just 90°C, demonstrating a significant reduction in the required operating temperature.
Tomoya Kawaguchi, a professor at Tohoku University’s Institute for Materials Research (IMR), notes the broader implications of the study. “Lithium is scarce and unevenly distributed, whereas magnesium is abundantly available, offering a more sustainable and cost-effective alternative for lithium-ion batteries.”
“Magnesium batteries, featuring the newly developed cathode material, are poised to play a pivotal role in various applications, including grid storage, electric vehicles, and portable electronic devices, contributing to the global shift towards renewable energy and reduced carbon footprints.”
Kawaguchi collaborated with Tetsu Ichitsubo, also a professor at IMR, who says, “By harnessing the intrinsic benefits of magnesium and overcoming previous material limitations, this research paves the way for the next generation of batteries, promising significant impacts on technology, the environment, and society.”
Ultimately, the breakthrough is a major step forward in the quest for efficient, eco-friendly energy storage solutions.
Tomoya Kawaguchi et al, Securing cation vacancies to enable reversible Mg insertion/extraction in rocksalt oxides, Journal of Materials Chemistry A (2024). DOI: 10.1039/D3TA07942B
Citation:
Unleashing disordered rocksalt oxides as cathodes for rechargeable magnesium batteries (2024, March 29)
retrieved 29 March 2024
from https://techxplore.com/news/2024-03-unleashing-disordered-rocksalt-oxides-cathodes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
If you want to read more Like this articles, you can visit our Science category.



