#The structural basis of focal adhesion kinase activation on lipid membranes unravelled
“#The structural basis of focal adhesion kinase activation on lipid membranes unravelled”
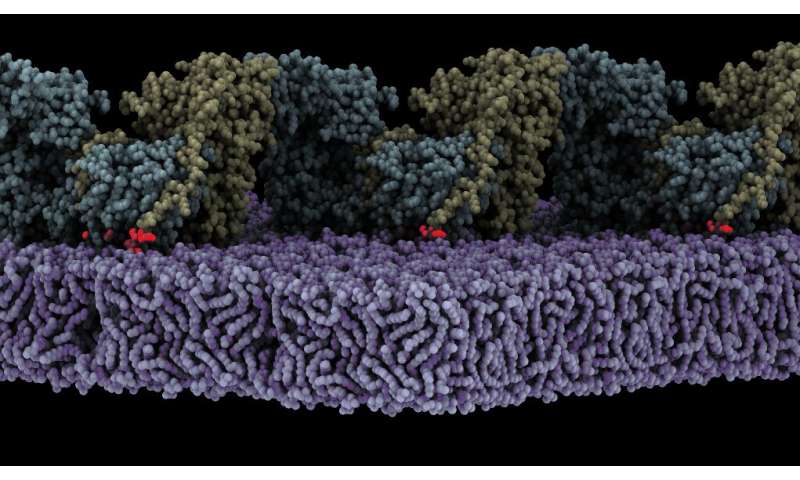
A research team led by Daniel Lietha has just published in The EMBO Journal the mechanistic details of the activation of the focal adhesion kinase (FAK) on lipid membranes. Lietha started this research during his work at the Spanish National Cancer Research Center (CNIO) and has culminated it in his current institution, the Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC).
FAK is a key protein ensuring controlled cell adhesion, proliferation, migration, and survival, which in cancer is often responsible for aberrant cell invasion, leading to metastatic cancers. In the cytosol, FAK adopts an autoinhibited state but is activated upon recruitment into focal adhesions, yet how this occurs or what induces the structural changes was unknown.
Lietha’s group have demonstrated that FAK is activated when it is localized to the cell membrane, where it interacts with specific phosphoinositide lipids. Now, the high-resolution structure of an oligomeric form of FAK bound to a lipid membrane has been obtained using Cryo-Electron Microscopy. The analysis of the structure shows that initial binding of FAK to the membrane causes steric clashes that release the kinase domain from autoinhibition, allowing it to undergo a large conformational change and interact itself with the membrane in an orientation that places the active site towards the membrane.
The structure also reveals that several interfaces align in the rearranged conformation to allow oligomerization of FAK on the membrane with a key phosphorylation site exposed, leading to autophosphorylation and, in turn, activation of FAK. Molecular dynamics simulations were carried out to understand the mechanism and dynamics of the process of autophosphorylation and subsequent activation on the membrane.
To validate the computational model, different mutants of FAK were generated carrying mutations at the observed interfaces. Extensive biochemical experiments were carried out to evaluate how the different mutations affect lipid binding, FAK autophosphorylation, and activation. Moreover, how the mutations affect FAK function in cancer cells was also studied, revealing that the uncovered mechanism is key for cancer cell invasion and proliferation.
More information:
Structural basis of Focal Adhesion Kinase activation on lipid membranes, EMBO Journal (2020). DOI: 10.15252/embj.2020104743
The structural basis of focal adhesion kinase activation on lipid membranes unravelled (2020, August 11)
retrieved 11 August 2020
from https://phys.org/news/2020-08-basis-focal-adhesion-kinase-lipid.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com




