#Stereoselective alkene synthesis with non-precious nickel catalysis

“#Stereoselective alkene synthesis with non-precious nickel catalysis”
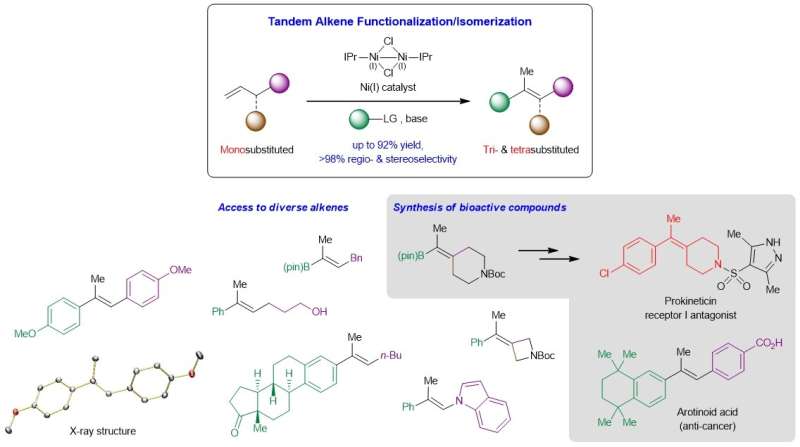
NUS chemists have developed a new way to access prized tri- and tetrasubstituted alkenes through a nickel-catalyzed tandem process involving a Heck reaction followed by carbon-carbon double bond (C=C) migration.
The development of reliable methods that afford stereochemically defined acyclic tri- and tetrasubstituted alkenes is a longstanding goal in organic synthesis. These highly substituted C=C bonds commonly reside in countless molecules of interest including organic materials and biologically active entities, and are key intermediates for further derivatization to a broader spectrum of high-value products. Existing reactions that convert carbonyl compounds or unsaturated carbon-carbon bonds to tri- and tetrasubstituted olefins often involve lengthy synthetic routes, exhibit limited functional group compatibility, suffer from unsatisfactory regio- or stereoselectivity control, and/or are not sufficiently general. One complication arises from the small energy difference between the cis and trans isomers of these highly substituted alkenes, which increases the difficulty of generating these compounds in high stereoselectivity.
A research team led by Prof Koh Ming Joo, from the Department of Chemistry, National University of Singapore in collaboration with Prof Osvaldo Gutierrez, from the University of Maryland, has conceived a tandem strategy that merges regiocontrolled Heck reaction and stereocontrolled C=C bond migration in a single step (see Figure). Mechanistic and computational studies showed that the reaction proceeds through a non-radical pathway, and that both the sizeable alkoxide base and N-heterocyclic carbene (NHC) ligand are crucial for the catalytic process.
Prof Koh said, “Our initial foray into this research area was to hypothetically ask how can we transform monosubstituted α-olefins, a highly abundant class of feedstock chemicals, to the more valuable but difficult-to-synthesize trisubstituted and tetrasubstituted analogs, in a single process. As it turns out, the solution to this was to design a tandem reaction that first reacts with the monosubstituted substrate, then isomerizes it to the desired product. A well-orchestrated control of regio- and stereoselectivity was paramount, which led us to discover the unique effectiveness of the NHC-ligated nickel catalysts.”
“We expect our new methodology to enhance the way in which many bioactive molecules are synthesized, and to serve as a blueprint for the design of catalytic tandem transformations to construct important building blocks from non-precious materials,” added Prof Koh.
The research team plans to exploit the insights obtained from this work to develop new tandem transformations to facilitate fine chemical synthesis.
Manipulating chain-walking in olefins using earth-abundant iron-based catalysts
Chen-Fei Liu et al, Olefin functionalization/isomerization enables stereoselective alkene synthesis, Nature Catalysis (2021). DOI: 10.1038/s41929-021-00658-2
Citation:
Stereoselective alkene synthesis with non-precious nickel catalysis (2021, August 10)
retrieved 10 August 2021
from https://phys.org/news/2021-08-stereoselective-alkene-synthesis-non-precious-nickel.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.




