#Q&A: Toward a new way of producing solar cells
“#Q&A: Toward a new way of producing solar cells”
Physicists from the University of Luxembourg together with international scientists have investigated the oxidation process of solar cell materials whose results could change the current way of producing solar cells. The study has been published in the renowned journal Nature Communications in July 2020.
Phase boundaries are critical points for the properties of materials. The research team has just found that when materials used for solar cells are near a phase boundary, the oxidation process creates much more damage than just the oxidation.
This publication is a result of a four-year research project and fruitful collaboration within the Department of Physics and Materials Science (DPhyMS) between the Laboratory for Energy Materials (LEM) led by Prof. Phillip Dale and the Laboratory for Photovoltaics (LPV) led by Prof. Susanne Siebentritt. The project was successfully conducted by Diego Colombara and Hossam Elanzeery who were at that time postdoctoral researcher and doctoral researcher at the University of Luxembourg, respectively.
The team explains in more details the project.
What is a phase boundary for solar cell materials?
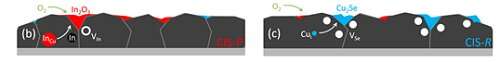
“When ice melts and becomes water, it crosses a phase boundary. In this case, it is the temperature that makes the material cross the phase boundary. In compound semiconductors, like copper indium selenide, which is used in solar cells, it is the composition that makes the material cross the phase boundary. In the ideal crystal, there is as much Cu as In, and when there is more Cu than In the material is in a different phase than when there is less Cu than In.”
How do you control this change?
“We can control this by the deposition process. It has been known for a long time, that when the material oxidizes, for example, when we leave it too long in the air, it forms an indium oxide. What we now found is: when the Cu-rich material oxidizes, it not only forms indium oxide but it also becomes too Cu-rich. So, the Cu must leave the material. And in doing so, it takes the selenium with it, forming new defects, the selenium vacancies. And those are bad for the solar cells. This insight is not only important for the way how we make solar cells. Selenide materials have other applications in data storage, lasing and communication. The findings will be relevant also to those other selenides or sulfides that show similar phase boundaries.”
How can we build better solar cells?
“We now know the root mechanism for the emergence of these damaging defects in our solar cells and have already found that it is possible to partially call off these defects once they are formed, by forcing an excess of selenium from the outside. Building on this knowledge base, we will design fabrication methods that prevent defect formation altogether, as part of our roadmap for more efficient solar energy conversion.”
More information:
Diego Colombara et al. Chemical instability at chalcogenide surfaces impacts chalcopyrite devices well beyond the surface, Nature Communications (2020). DOI: 10.1038/s41467-020-17434-8
Q&A: Toward a new way of producing solar cells (2020, July 20)
retrieved 20 July 2020
from https://phys.org/news/2020-07-qa-solar-cells.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com




