#Optimizing gas mixtures for hydrogen storage in clathrate hydrates

“#Optimizing gas mixtures for hydrogen storage in clathrate hydrates”
In our ongoing quest to transform into a more eco-friendly society, hydrogen (H2) is heralded as the clean fuel of tomorrow. Because H2 can be produced from water (H2O) without generating carbon emissions, developing H2-compatible technologies has become a top priority. However, the road ahead is bumpy, and many technical limitations must be ironed out.
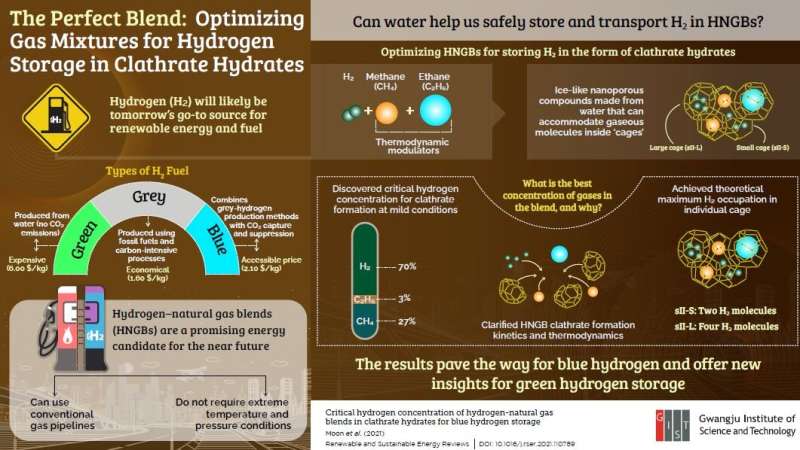
“Hydrogen is the smallest molecule in nature, and finding feasible ways to store it is a critical issue to realize a hydrogen economy,” states Associate Professor Youngjune Park from the Gwangju Institute of Science and Technology (GIST) in Korea. Unlike hydrocarbons, pure H2 must be stored at an extremely high pressure (>100 atmospheres) or low temperature (20 °C). Naturally, this represents a huge economic barrier for H2 storage. But what if we could trap H2 inside ice-like crystals to make storage and transportation less demanding?
These molecular cages exist in nature and are called “clathrate hydrates.” They are solid water-based compounds with cavities that can accommodate various molecules. Dr. Park’s group at GIST has been researching the use of clathrate hydrates as vessels for H2 storage. However, the enclathration of pure H2 is still a slow process that also requires extreme temperature and pressure conditions.
In a recent study published in volume 141, the May 2021 print issue of Renewable and Sustainable Energy Reviews, Dr. Park’s group explored a feasible solution to this problem. Instead of trying to form clathrate hydrates out of pure H2, previous researchers have suggested mixing it with natural gas, which was experimentally shown to promote enclathration at milder conditions. To improve upon this strategy, the team of GIST scientists set out to find the best hydrogen-natural gas blend (HNGB) for the energy-efficient formation of clathrate hydrates. To this end, they systematically investigated clathrate hydrates produced from HNGBs with different concentrations of methane, ethane, and hydrogen. They carefully analyzed the clathrate formation kinetics and structure and the distribution of trapped molecules.
The team was able to identify the precise gas concentrations at which point methane and ethane, acting as thermodynamic modulators, best enhance the H2 storage capacity of HNGB hydrates. Even at moderate pressure and temperature conditions (100 atmospheres and 8 °C, respectively), the scientists achieved the maximum theoretical H2 storage possible for two types of clathrate hydrate cages: two and four H2 molecules in small and large cages, respectively. This feat had not been reported before, and the unprecedented findings of this study could thus help in the design of HNGB hydrate storage media.
Dr. Park observes, “Clathrate hydrates and HNGBs could provide a reasonable mid-term solution for storing what is known as ‘blue’ hydrogen, which is hydrogen produced using fossil fuel-based technology but with minimal CO2 emissions.” Today, blue hydrogen is three times cheaper to produce than eco-friendly ‘green’ hydrogen. Therefore, the results of this study may help ease the gradual transition away from fossil fuels towards hydrogen, which is our key to a sustainable future.
Hydrogen-natural gas hydrates harvested by natural gas
Seokyoon Moon et al, Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage, Renewable and Sustainable Energy Reviews (2021). DOI: 10.1016/j.rser.2021.110789
Provided by
Gwangju Institute of Science and Technology
Citation:
The perfect blend: Optimizing gas mixtures for hydrogen storage in clathrate hydrates (2021, May 17)
retrieved 17 May 2021
from https://phys.org/news/2021-05-blend-optimizing-gas-mixtures-hydrogen.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.




