#Novel technique seamlessly converts ammonia to green hydrogen

“#Novel technique seamlessly converts ammonia to green hydrogen”
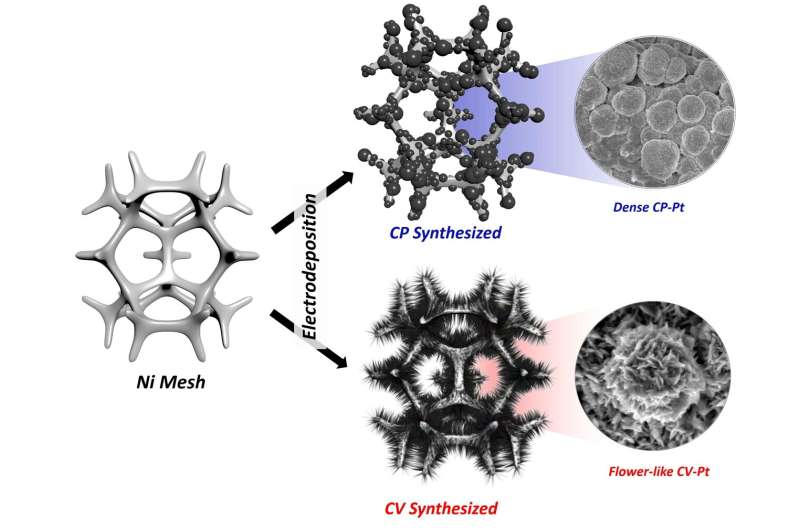
A research team led by Professor Guntae Kim in the School of Energy and Chemical Engineering at UNIST has announced a breakthrough in technology that efficiently converts liquid ammonia into hydrogen. Their findings have also attracted significant attention from academic research communities owing to its new analysis protocol, capable of finding optimal process environments.
In this study, the research team succeeded in producing green hydrogen (H2) in large quantities with a purity of nearly 100 percent by decomposing liquid ammonia (NH3) into electricity. Besides, according to the research team, such method consumed three times less power than hydrogen made using electrolysis of water.
Ammonia has emerged as an attractive potential hydrogen carrier due to its extremely high energy density, and ease of storage and handling. Moreover, the electrolysis of ammonia to produce nitrogen and hydrogen only requires an external voltage of 0.06 V theoretically, which is much lower than the energy needed for water electrolysis (1.23 V), noted the research team.
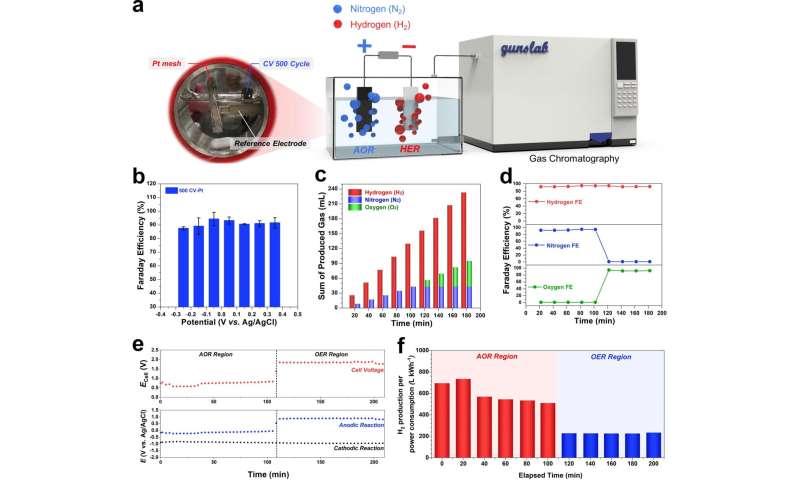
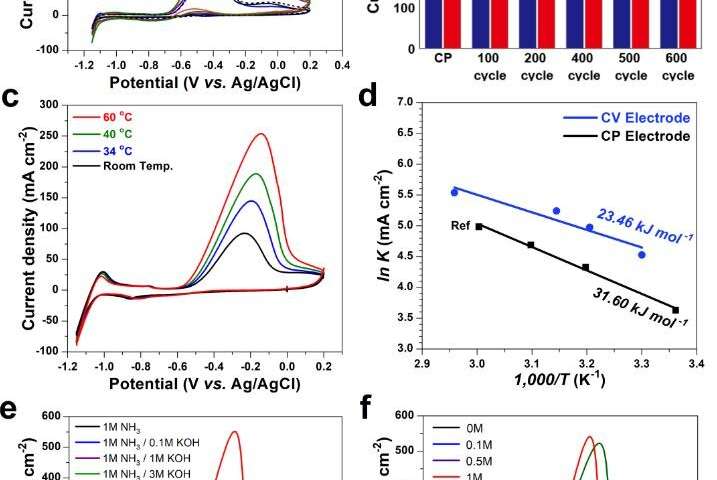
In this study, the research team propose a well-established procedure using in operando gas chromatography that enables us to reliably compare and evaluate the new catalyst for ammonia oxidation. According to the research team, with the protocol, they could distinguish in detail the competitive oxidation reaction between the ammonia oxidation and oxygen evolutionreactions with real-time monitoring.
-
Credit: Ulsan National Institute of Science and Technology
-
Credit: Ulsan National Institute of Science and Technology
With the use of flower-like electrodeposited Pt catalyst, researchers have efficiently produced hydrogen with less power consumption of 734 LH2 kW h−1, which is significantly lower than that of the water-splitting process (242 LH2 kW h−1). “The use of this rigorous protocol should help to evaluate the practical performances for ammonia oxidation, thus enabling the field to focus on viable pathways towards the practical electrochemical oxidation of ammonia to hydrogen,” noted the research team.
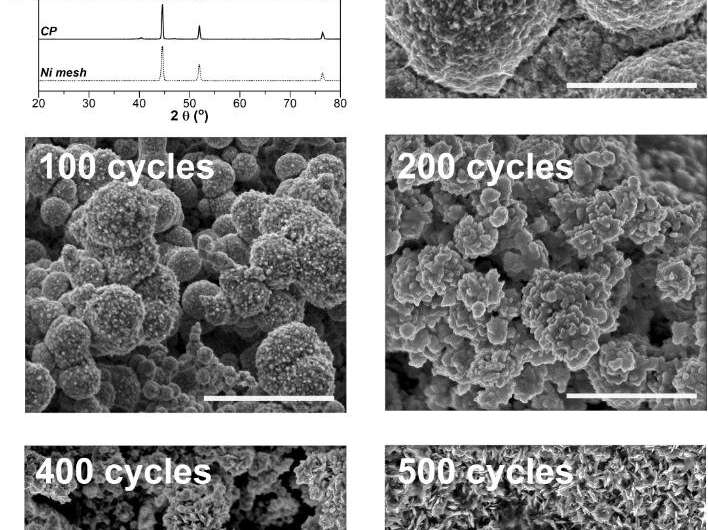
This study has been co-authored by Minzae Lee, Myung-gi Seo, Hyung-Ki Min, and Youngheon Choi from Lotte Chemical R&D Center, respectively. Their work has also been featured on the inside back cover of Journal of Material Chemistry A, which was made available online in March 2021 ahead of final publication in May 2021.
Ammonia decomposition for hydrogen economy, improvement in hydrogen extraction efficiency
Yejin Yang et al, A rigorous electrochemical ammonia electrolysis protocol with in operando quantitative analysis, Journal of Materials Chemistry A (2021). DOI: 10.1039/D1TA00363A
Citation:
Novel technique seamlessly converts ammonia to green hydrogen (2021, August 12)
retrieved 12 August 2021
from https://phys.org/news/2021-08-technique-seamlessly-ammonia-green-hydrogen.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.




