#Designing a freestanding, supercharged polypeptide proton-conducting membrane
“#Designing a freestanding, supercharged polypeptide proton-conducting membrane”
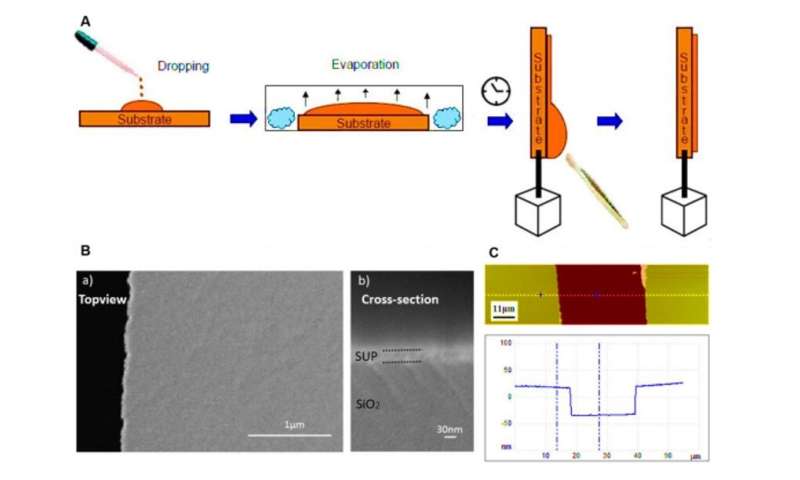
Developing proton-conducting protein materials
In the polypeptide backbone of the proton-conducting membrane, the hydrophilic (water-loving) charged moieties served as proton carriers. The team studied the proton-conducting performance of these unfolded systems to obtain freestanding membranes and perfected the structural design by amalgamating silk-like β-sheet structures with anionic SUPs to form self-assembled nanostructures. The team decorated the surfaces with dense carboxylic acid groups for hydration, proton dissociation and to form proton conduction pathways. The mechanically stable and freestanding membrane surpassed hitherto reported transport properties of protein-based systems for outstanding proton conductivity.
The team derived the supercharged proteins from elastin; explored previously for applications of protein engineering and interface modification. They introduced glutamic acid (abbreviated Glu or E), which can be easily deprotonated under physiological conditions into the X site of the protein sequence, to form unstructured negatively supercharged polypeptides (SUP-Es). Then they constructed three different variants of supercharged polypeptides known as E72, HC_E35 and DC_E108. Ma et al. used electrochemical impedance spectroscopy (EIS) with gold interdigitated electrodes (IDEs) to evaluate thin-film proton conduction and measured proton transport as a function of relative humidity. When the humidity increased to 90 percent, proton translocation improved due to absorption of a large number of water molecules via the carboxylic acid (-COOH) groups of the material. Besides relative humidity, they also investigated proton conduction relative to charge carrier density for the specimens of interest. By tuning the charge density of the disordered proteins, Ma et al. successfully controlled proton conductance behavior of proteins within films. Due to the high stability and uniformity of the thin films made of SUPs, the setup did not show signs of defects.
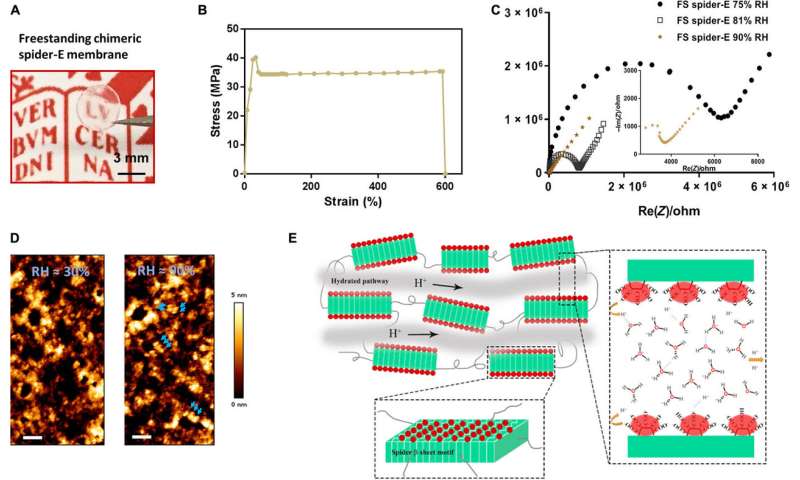
In this way, Chao Ma and colleagues applied rational molecular de novo design and engineering to achieve a bioinspired protein-derived bulk material with robust properties of proton conduction and excellent mechanical stability. They tested the surface modifications using a range of biophysical tools. The team developed the new generation, bioinspired bulk material and explored successive sequence designs to offer a promising platform for applications in biotechnology and envision the use of such materials for proton transport in miniaturized biofuel cells of the future.
More information:
Chao Ma et al. De novo rational design of a freestanding, supercharged polypeptide, proton-conducting membrane, Science Advances (2020). DOI: 10.1126/sciadv.abc0810
I. N. Watt et al. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria, Proceedings of the National Academy of Sciences (2010). DOI: 10.1073/pnas.1011099107
Jeff A. Hurd et al. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework, Nature Chemistry (2009). DOI: 10.1038/nchem.402
© 2020 Science X Network
Designing a freestanding, supercharged polypeptide proton-conducting membrane (2020, July 24)
retrieved 24 July 2020
from https://phys.org/news/2020-07-freestanding-supercharged-polypeptide-proton-conducting-membrane.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com




