#Point-of-care test developed for tumor marker in human saliva based on lanthanide nanoprobes

“#Point-of-care test developed for tumor marker in human saliva based on lanthanide nanoprobes”
Salivary assay, emerging as a non-invasive alternative to blood assay in clinic analysis, holds great promise for early-stage cancer diagnostics with advantages of low cost, easy collection and facile processing. Therefore, point-of-care (POC) detection of tumor markers in the saliva is urgently demanded.
However, the salivary assay has been severely hindered by the limitation of inadequate sensitivity in current commercial assay kits because of the much lower level of tumor markers in saliva than in human serum.
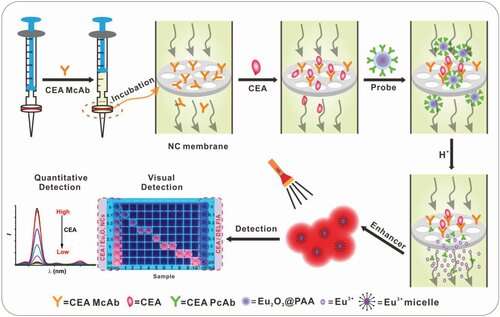
In a study published in Advanced Science, a research group led by Prof. Chen Xueyuan from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences developed a unique lab-in-syringe strategy for rapid and ultrasensitive detection of tumor markers in saliva based on lanthanide nanoprobes.
The researchers employed Eu2O3 nanocrystals as bioprobes, which can be easily dissolved in acidic enhancer solution and transformed into a large number of highly luminescent Eu3+ micelles. Meanwhile, they utilized a disposable syringe filter equipped with nitrocellulose membrane as bioassay platform, which facilitated the accomplishment of detection process within 10 min.
By ingenious integration of dissolution enhanced luminescent bioassay strategy and the miniaturized detection device, the researchers demonstrated the feasibility and reliability for the direct quantitation of tumor marker like carcinoembryonic antigen (CEA) in patient saliva samples with a detection limit down to 1.47 pg/mL (7.35 fM).
More importantly, by virtue of such an excellent luminescence-amplification strategy, the researchers can visually detect the photoluminescence intensity change above 0.1 ng/mL (0.5 pM) of CEA by naked eyes to qualitatively evaluate its level in saliva. The whole detection process is easy to operate, which is highly beneficial for cancer diagnostics by ordinary people at home.
These findings revealed the great potential of the proposed general strategy in practical home self-monitoring of trace amount of tumor markers in saliva, which may accelerate the exploitation of lanthanide nanoprobes for POC bioassay of diverse disease markers in complex biological fluids.
Direct detection of circulating tumor cells in blood samples
Shanyong Zhou et al. Ultrasensitive Point‐of‐Care Test for Tumor Marker in Human Saliva Based on Luminescence‐Amplification Strategy of Lanthanide Nanoprobes, Advanced Science (2021). DOI: 10.1002/advs.202002657
Citation:
Point-of-care test developed for tumor marker in human saliva based on lanthanide nanoprobes (2021, January 21)
retrieved 21 January 2021
from https://phys.org/news/2021-01-point-of-care-tumor-marker-human-saliva.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.



