#The exhaust gas from a power plant can be recovered and used as a raw reaction material
“#The exhaust gas from a power plant can be recovered and used as a raw reaction material”
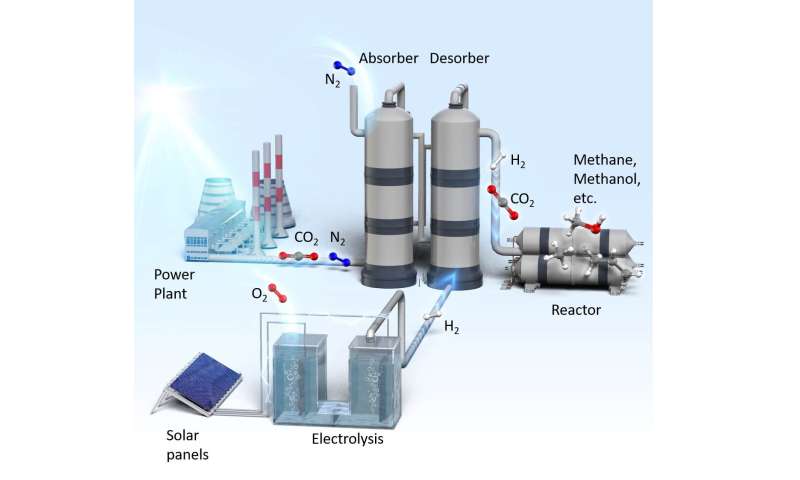
A research group at Nagoya University has developed a new technology that can drastically conserve the energy used to capture carbon dioxide (CO2), one of the greenhouse gases, from facilities such as thermal power plants. Conventionally, a significant amount of energy (3 to 4 GJ/ton-CO2) or high temperatures exceeding 100 deg.C has been required to capture CO2 from gases exhausted from a concentrated source, and there are expectations of the development of CO2capture technology that consumes less energy.
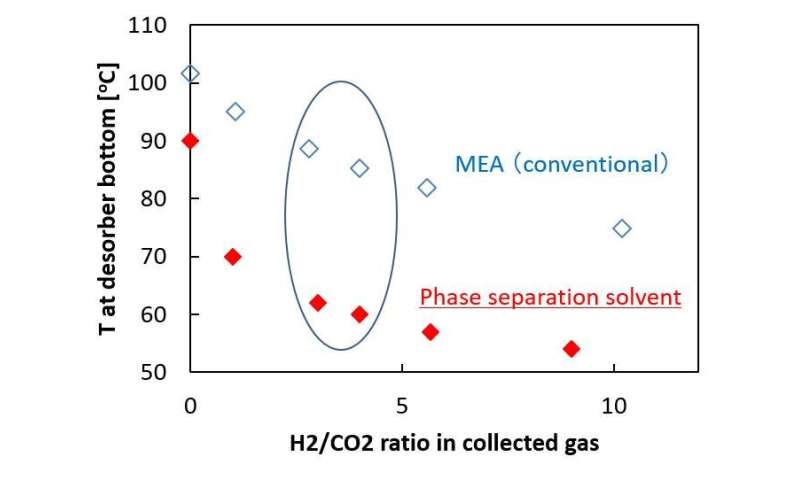
The research group led by Assistant Professor Hiroshi Machida has developed an unprecedented CO2 capture technology, namely H2 stripping regeneration technology1), in which hydrogen (H2) gas is supplied to the regeneration tower (desorber)2). It is indicated in this research that, with the implementation of this new technology, combustion exhaust gas can be replaced by CO2/H2 gas at lower temperatures (85 deg.C) than those used in conventional technology. The further reduction of energy can be achieved when it is combined with technologies such as those involved in the promotion of exhaust heat utilization and recovery of reaction heat.
This new technology can exhibit the world’s highest energy-saving performance (i.e., separation and collection of energy required is less than 1 GJ/ton-CO2 when a desorber temperature is 60 deg.C) when it is combined with the phase-separation solvent that this research group has also developed.
This technology is expected to be applicable to value-added material production such as the syntheses of methane, methanol, gasoline, etc., from CO2 in the combustion exhaust gas and H2 from renewable energy, and is expected to contribute to carbon recycling.
(1) H2 stripping regeneration technology
In the conventional process to synthesize fuel or chemicals from CO2 and renewable H2, pure CO2 is collected and is then mixed with H2 before being supplied to the reduction reactor. In the H2 stripping regeneration technology, H2 gas is supplied at the bottom of the desorber. As a result, CO2 partial pressure in the desorber is lowered, which promotes regeneration and lowers the regeneration temperature. The mixture of CO2 and H2 gases collected from the head of the desorber is supplied directly to the synthesis reactor.
(2) Regeneration tower (Desorber)
In the amine absorption method, CO2 absorption and regeneration towers (i.e., absorber and desorber) are used to separate and collect CO2 in the exhaust gas mixture from facilities like power plants. Gases such as N2and O2, in addition to CO2, are included in the combustion exhaust gas from these facilities and pure CO2 gas is collected with this amine absorption method. Only the CO2 gas is absorbed in the absorber by amine solution, and it is then heated in the desorber to regenerate pure CO2 gas. In other words, only CO2 gas can be extracted from the mixture of gases.
More information:
Hiroshi Machida et al. Energy-Saving CO2 Capture by H2 Gas Stripping for Integrating CO2 Separation and Conversion Processes, ACS Sustainable Chemistry & Engineering (2020). DOI: 10.1021/acssuschemeng.0c02459
Provided by
Japan Science and Technology Agency
The exhaust gas from a power plant can be recovered and used as a raw reaction material (2020, June 19)
retrieved 19 June 2020
from https://phys.org/news/2020-06-exhaust-gas-power-recovered-raw.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com




