#Scientists reveal a potential new approach to treating liver cancer

Table of Contents
“Scientists reveal a potential new approach to treating liver cancer”
Scientists at the National Institutes of Health and Massachusetts General Hospital in Boston have uncovered a potential new approach against liver cancer that could lead to the development of a new class of anticancer drugs. In a series of experiments in cells and mice, researchers found that an enzyme produced in liver cancer cells could convert a group of compounds into anticancer drugs, killing cells and reducing disease in animals.
The researchers suggest that this enzyme could become a potential target for the development of new drugs against liver cancers, and perhaps other cancers and diseases as well.
“We found a molecule that kills cells in a rare liver cancer in a unique way,” said translational scientist Matthew Hall, Ph.D., one of the leaders of the work at NIH’s National Center for Advancing Translational Sciences (NCATS). “It emerged from a screening to find molecules that selectively kill human liver cancer cells. It took a lot of work to figure out that the molecule is converted by an enzyme in these liver cancer cells, creating a toxic, anticancer drug.”
Hall, Nabeel Bardeesy, Ph.D., a liver cancer specialist at Massachusetts General Hospital and their colleagues reported their results March 13 in Nature Cancer.
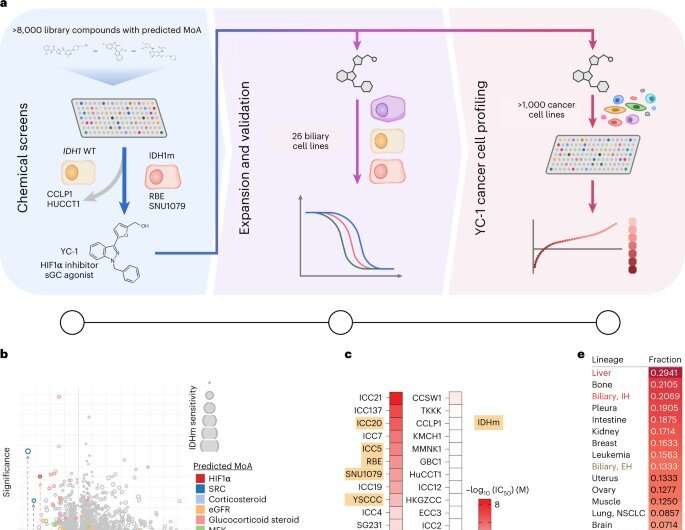
The finding stems from a collaboration between Massachusetts General Hospital and NCATS researchers. Bardeesy was originally studying cholangiocarcinoma, a type of liver cancer that affects the bile duct. The cancer is characterized by mutations in the IDH1 enzyme. Bardeesy’s team wanted to find compounds and drugs that might be effective against the IDH1 mutation. Through a collaboration with NCATS, Hall and other NCATS scientists rapidly tested thousands of approved drugs and experimental cancer agents for their effectiveness in killing cholangiocarcinoma cells, with IDH1 as a target.
They found several molecules, including one called YC-1, could kill the cancer cells. Yet, when they looked to see how YC-1 was working, they discovered the compound wasn’t affecting the IDH1 mutation.
The Massachusetts researchers showed that the liver cancer cells made an enzyme, SULT1A1. The enzyme activated the YC-1 compound, making it toxic to tumor cells in cancer cell cultures and mouse models of liver cancers. In the animal models treated with YC-1, the liver tumors either had reduced growth or shrank. Conversely, the researchers found no changes in tumors treated with YC-1 in animals with cancer cells lacking the enzyme.
The researchers examined other databases of drug screening results in compound and drug libraries to match drug activity with SULT1A1 activity. They also looked at a large National Cancer Institute database of anticancer compounds for additional possibilities to test for their activity with the enzyme.
They identified several classes of compounds that relied on SULT1A1 for their tumor-killing activity. Using computational methods, they predicted other compounds that also likely were dependent on SULT1A1.
“Once we found SULT1A1 activated YC-1, it led us to ask, “What other compounds are active and can kill cells by the same mechanism?” Hall said. “Can we identify other compounds that were being developed and demonstrate that they were also active because of SULT1A1 activation? The answer was yes. We found other compounds with the same mechanism of action as YC-1.”
The scientists suggest these findings have broader implications for developing new anticancer drugs. “We think these molecules have the potential to be an untapped class of anticancer drugs that depend on SULT1A1 for their activity against tumors,” Bardeesy said.
The researchers see YC-1 and similar molecules as prototypes for developing compounds that could be effective against important proteins on cells. Modifying different parts of these molecules could make them more specific for such proteins. The researchers point to the creation of a “toolkit of SULT1A1-activated molecules” that could affect many different targets.
Such a toolkit is comprised of hundreds of known molecules. In theory, the toolkit covers many types of enzymes, called sulfotransferases, that are active in different tissues in the body. For example, in addition to SULT1A1, the human sulfotransferase SULT4A1 is active in the brain. It can activate a subset of the molecules in the toolkit. This might be useful in developing drugs specific for brain cancers.
“We knew SULT1A1-dependent drugs had already been identified,” Bardeesy said. “Our results suggest there could be other SULT1A1-dependent compounds with ranges of different targets. Identifying such compounds and targets on cells could have potential implications for developing other types of small molecules and drugs, not just limited to these cancers. This might become a new approach for some diseases.”
More information:
Lei Shi et al, SULT1A1-dependent sulfonation of alkylators is a lineage-dependent vulnerability of liver cancers, Nature Cancer (2023). DOI: 10.1038/s43018-023-00523-0
Citation:
Scientists reveal a potential new approach to treating liver cancer (2023, March 13)
retrieved 13 March 2023
from https://medicalxpress.com/news/2023-03-scientists-reveal-potential-approach-liver.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.



