#Scientists explore lipid metabolism with simulations and experiments

“#Scientists explore lipid metabolism with simulations and experiments”
In humans and animals, fat cells produce and store fat in special organelles, which are specialized subunits within the cell, called lipid droplets. The lipid droplets make up the largest part of the volume of these fat cells, also known as adipocytes. Adipocytes fulfill vital tasks protecting our organs, as well as parts of the body that are sensitive to cold, and they serve as a source of reserve energy. But when we eat too much fat-rich food, the adipocytes and thus the fat deposits grow excessively, leading, in the worst-case scenario, to the development of pathologies such as obesity and obesity-related diseases. In contrast, certain individuals develop a disease because no lipid droplets are formed at all. The associated clinical picture is called lipodystrophy, in which the affected individuals are unable to store lipids and thus become seriously ill.
In addition to adipocytes, every single cell of the human organism contains lipid droplets. The organelles serve mainly as energy suppliers, for example during cell growth, but they are also involved in lipid metabolism inside the cells.
“The role of lipid droplets is critical. And when lipid storage is poorly regulated, pathologies such as obesity, lipodystrophy and cancer develop,” says Stefano Vanni, SNSF professor at the University of Fribourg. Proper regulation of lipid storage is therefore essential for every single cell in the entire organism.
Simulating lipid metabolism
Vanni and his research team are investigating how these lipid droplets store lipids in cells. The scientists run coarse-grain molecular dynamics simulations—where chemical groups are represented by individual beads to simplify the representation of complex systems—on the CSCS supercomputer “Piz Daint” to better understand the mechanisms involved in lipid storage at the molecular level. Their research findings on lipid metabolism were recently published in both eLife and the Proceedings of the National Academy of Sciences.
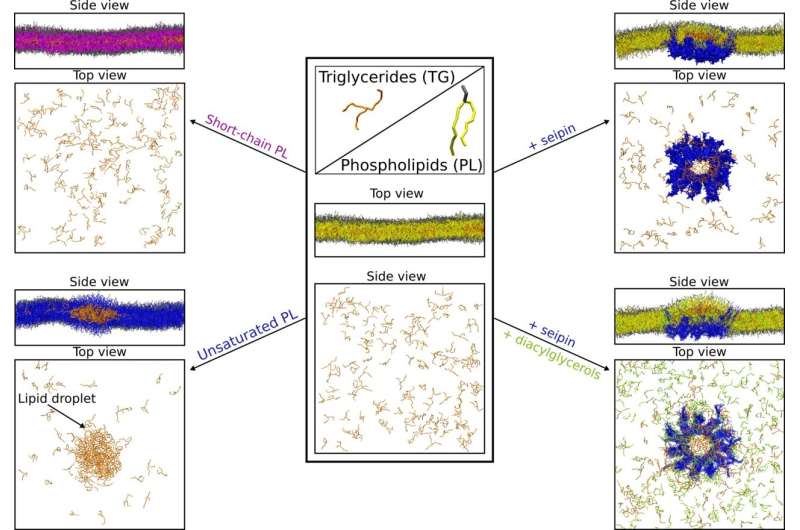
In the first study, they investigated how lipid droplets are formed. As was known from previous studies, the droplets form after accumulation of fat between the two leaflets of the endoplasmic reticulum membrane, another organelle of the cell. The endoplasmic reticulum expands throughout the entire cell volume and, among other things, plays an important role in lipid metabolism. However, exactly how the formation of lipid droplets is connected to the endoplasmic reticulum and how the process is regulated was still unclear.
“One difficulty in simulating lipid droplets is their magnitude: With a size of 50 to 100 nanometers, they are large objects compared to individual proteins,” says Vanni. This means that very large computing resources are needed for their simulations, but, in turn, the results obtained can be directly compared with in vivo observations of the organelles under the microscope. The scientists therefore compared their conclusions from the simulations of how lipid droplets are formed to the results of an experimental research group also based at the University of Fribourg. Specifically, the experimental group investigated the effect of gene-modification on lipid droplet formation in yeast cells, observed in vivo using fluorescence microscopy.
Different types of lipids ensure proper lipid droplet formation
By combining in silico and in vivo experiments, the researchers were able to show that the so-called phospholipids, the lipids that constitute the membranes of the endoplasmic reticulum, either promote or prevent the aggregation of triglycerides, which are the main form of fat in lipid droplets. According to the results, phospholipids with monounsaturated fatty acids in their structure seem to favor the formation of lipid droplets. However, the opposite happens when short saturated fatty acids are present in the phospholipids: Less lipid droplets are formed, and the free triglycerides move into the endoplasmic reticulum membrane instead of accumulating in lipid droplets.
Based on their findings, the researchers conclude that lipid droplet formation removes stress from the endoplasmic reticulum: By driving free triglycerides from the two leaflets of the endoplasmic reticulum membrane into lipid droplets, the cells are protected from high fatty acid levels (lipotoxicity) that could ultimately lead to cell death.
How the protein seipin controls lipid droplets building
With further simulations, Vanni and his co-workers also investigated the role of the protein seipin, since recent works have shown that lipid droplets formation always occurs at the sites where this protein is present. The exact role of seipin in the process of lipid droplets formation is still unclear—the protein was discovered only two decades ago in connection with the above-mentioned lipodystrophy, says Vanni, as specific mutations in the protein can cause the disease. One of seipin’s mysteries, according to Vanni, is that when the protein is knocked out the lipid droplets do not simply disappear. Rather, either very large or very small lipid droplets are formed, making it difficult to understand the protein’s function.
The simulations that Vanni’s group performed show that seipin traps and accumulates triglycerides within its ring-shaped structure and—surprisingly for the researchers—also the precursors of these types of fats, namely diacylglycerols. However, if mutations are introduced in the specific seipin region that traps triglycerides, the formation of the lipid droplets is hindered. The study also suggests that the protein seipin remodels certain sites of the endoplasmic reticulum, preparing them for the formation of lipid droplets. With a better understanding of the mechanisms underlying fat metabolism, researchers hope to explain why, for example, polyunsaturated fats are healthier than unsaturated fats, and why the latter should be reduced in our diet. In addition, the results could help to improve the understanding of metabolic diseases related to fat storage and thus aid in the development of drugs or dietary products.
Valeria Zoni et al, Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets, eLife (2021). DOI: 10.7554/eLife.62886
Valeria Zoni et al, Seipin accumulates and traps diacylglycerols and triglycerides in its ring-like structure, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2017205118
Citation:
Scientists explore lipid metabolism with simulations and experiments (2021, June 10)
retrieved 10 June 2021
from https://phys.org/news/2021-06-scientists-explore-lipid-metabolism-simulations.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.


