#Researchers observe new isotope of fluorine

“#Researchers observe new isotope of fluorine”
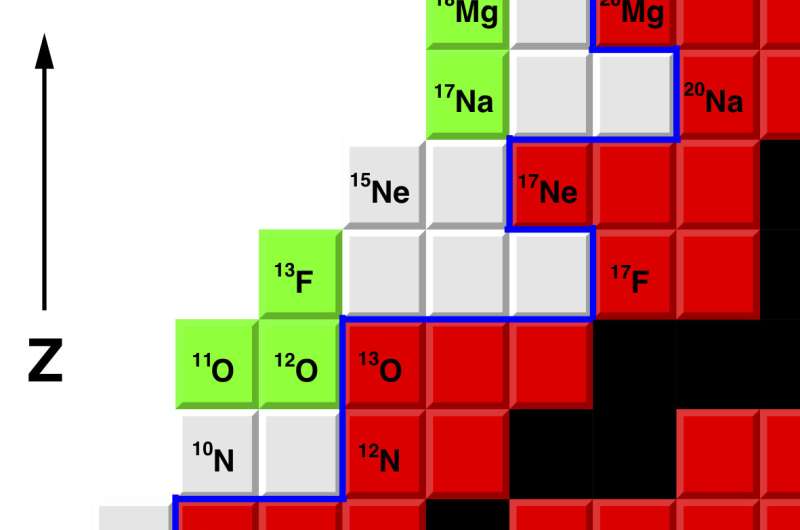
Researchers at Washington University in St. Louis reported the first observations of a new form of fluorine, the isotope 13F, described in the journal Physical Review Letters.
They made their discovery as part of an experiment conducted at the National Superconducting Cyclotron Laboratory at Michigan State University (MSU).
Fluorine is the most chemically reactive element on the periodic table. Only one isotope of fluorine occurs naturally, the stable isotope 19F. The new isotope, 13F, is four neutrons removed from the proton drip line, the boundary that delimits the zone beyond which atomic nuclei decay by the emission of a proton.
Robert J. Charity, research professor of chemistry in Arts & Sciences, and Lee G. Sobotka, professor of chemistry and of physics, worked in collaboration with groups from MSU, Western Michigan University and University of Connecticut to make this discovery.
“Study of exotic nuclei with such large excesses of neutrons or protons is of considerable interest in understanding the synthesis of elements, even though their lifetimes are extremely short,” Charity said. “Many of these isotopes have exotic properties.”
The isotope 13F is the fifth new isotope that Charity and Sobotka have discovered together.
“All the new isotopes are very proton-rich and unstable to the emission of protons,” Charity said. “The highest-energy protons inside these isotopes can tunnel through the Coulomb barrier and escape.”
The initial purpose of the experiment, Charity said, was to make a new isotope of oxygen, dubbed “featherweight oxygen,” a technical achievement previously reported in Physical Review Letters. After making that discovery, the researchers went through their data again with great care and teased out evidence for 13F.
The new isotope of fluorine was created via a charge-exchange reaction with a beam of 13O. (A neutron in the 13O is removed and replaced by a proton.)
“Such charge-exchange reactions have not typically been used for the creation of the very proton-rich isotopes in the past,” Charity said. “However, we are already planning a search for another new isotope using this reaction mechanism.”
A single proton can make a world of difference
R. J. Charity et al, Observation of the Exotic Isotope F13 Located Four Neutrons Beyond the Proton Drip Line, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.126.132501
Citation:
Researchers observe new isotope of fluorine (2021, March 30)
retrieved 31 March 2021
from https://phys.org/news/2021-03-isotope-fluorine.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.




