#Novel hydrogen fuel purification membrane paves the way for greener future

“#Novel hydrogen fuel purification membrane paves the way for greener future”
Hydrogen has been hailed as the ‘fuel of the future’ owing to several reasons. First, compared to the conventionally used hydrocarbons, hydrogen exhibits higher energy yield. Second, the commercial use of hydrogen fuel, which yields only water as a byproduct, would help mitigate the imminent global warming crisis by reducing the use of exhaustible and polluting fossil fuels. Thus, ongoing research has been focusing on efficient and environment-friendly ways to produce hydrogen fuel.
Solar hydrogen production through a photoelectrochemical (PEC) water-splitting reaction is an attractive ‘green’ method of hydrogen fuel production, owing to its potential for high conversion efficiency, low operating temperatures, and cost-effectiveness. However, efficient separation of hydrogen gas from a mixture of gases (called syngas) under different environmental conditions, has proven to be a challenge. A recent paper published in the journal Separation and Purification Technology seeks to address this challenge. In this study, a group of researchers from Nagoya Institute of Technology, Japan, led by Professor Yuji Iwamoto, in collaboration with researchers in France, successfully characterized a novel membrane that allows highly selective separation of hydrogen gas generated from the PEC reaction. Prof. Iwamoto says, “Membrane separation is attractive as a low-cost hydrogen gas purification technology. However, current techniques face several challenges, for example, water-induced swelling with polymer membranes and lower hydrogen permeance with metal, polymer, and supported liquid membranes. ”
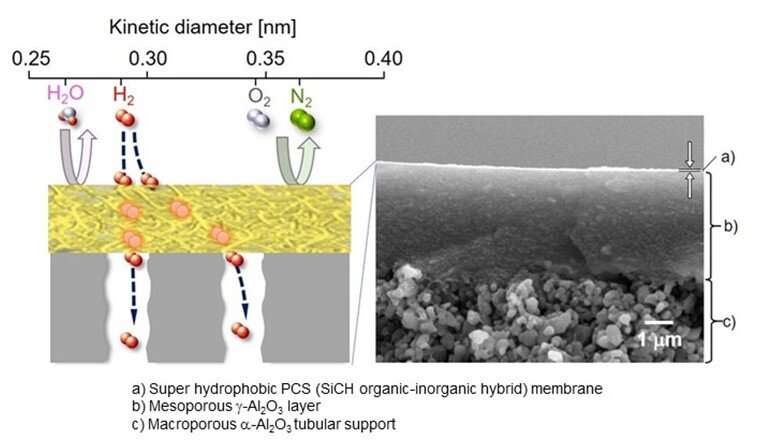
The researchers first developed an organic-inorganic hybrid polymeric membrane, primarily consisting of a polymer called polycarbosilane (PCS) formed on an aluminum oxide (Al2O3)-based porous support. Prof. Iwamoto further explains, “By using high-molecular-weight PCSs with a melting point above 200°C, we showed that a superhydrophobic PCS membrane could be deposited on a mesoporous γ-Al2O3-modified macroporous α-Al2O3 tubular support. ”
After successfully developing the PCS membrane, the researchers tested it under PEC reaction conditions. As hypothesized, the PCS membrane showed high hydrophobicity. Moreover, under the flow of a simulated highly humid gas mixture at 50°C, the PCS membrane exhibited excellent hydrogen selectivity. Further analysis revealed that the preferential hydrogen permeation through the PCS membrane was governed by the “solid state diffusion” mechanism. Overall, irrespective of the ambient environmental conditions provided, the PCS membrane exhibited efficient hydrogen gas separation.
With the development and characterization of this new PCS membrane, it is inevitable that its commercial adoption will not just facilitate the use of hydrogen fuel for energy needs but also curb the use of non-renewable fossil fuels. Prof. Iwamoto concludes, “With this technological development, we expect great progress in environmental-friendly and sustainable hydrogen production.”
Let’s hope that the use of PCS membrane is a step towards a hydrogen-based society.
Novel two-polymer membrane boosts hydrogen fuel cell performance
Miwako Kubo et al, Superhydrophobic polycarbosilane membranes for purification of solar hydrogen, Separation and Purification Technology (2020). DOI: 10.1016/j.seppur.2020.117998
Citation:
Novel hydrogen fuel purification membrane paves the way for greener future (2021, March 8)
retrieved 8 March 2021
from https://phys.org/news/2021-03-hydrogen-fuel-purification-membrane-paves.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.




