#New affinity purification technique for therapeutic proteins
“#New affinity purification technique for therapeutic proteins”
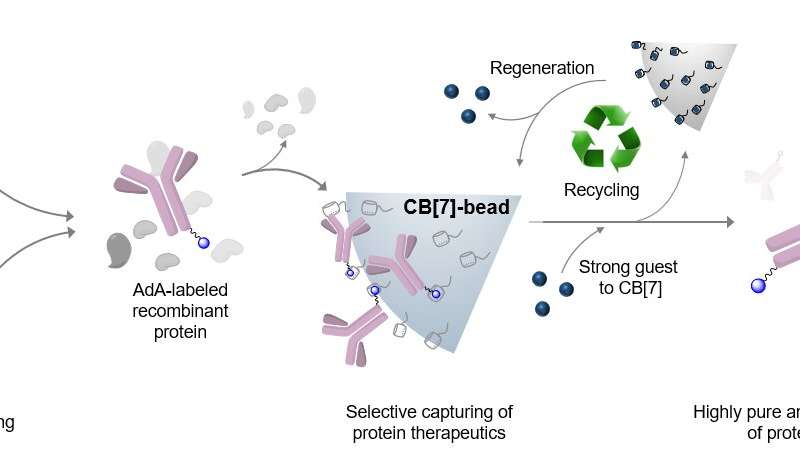
Professor Kimoon Kim’s research group at POSTECH has developed a highly pure and efficient technique for purifying antiviral and anti-cancer protein therapeutics using molecular affinity interaction.
With COVID-19 showing no signs of slowing down around the world, the development of a vaccine seems to be the sole solution to end the pandemic. However, even if a vaccine is developed, the task of manufacturing well-refined drug molecules, which treat a number of other comorbid conditions, remains elusive. Besides COVID-19, there are growing concerns for developing a source treatment protocol to deal with new infectious diseases that may find their way into our lives.
A joint research team made up of Professor Kimoon Kim (POSTECH University Professor of Department of Chemistry at POSTECH, Director of Center for Self-assembly and Complexity (CSC) and of Institute for Basic Science (IBS)) and Dr. Kyeng Min Park (Research fellow and Group leader at CSC and IBS) have together developed a source technology using the affinity interaction of cucurbiturils to purify recombinant therapeutic proteins used as antiviral drugs or as anti-cancer agents with high efficiency and high purity. The research findings were published online in Nature Biomedical Engineering on July 20.
Recombinant DNA technology uses biotechnological techniques to insert genes from one organism into another and activate them to change its genetic trait. Using this method, a vaccine can be developed to exterminate pathogenic microorganisms or weaken their toxicity by expressing all or part of the proteins. For the mass-production of the hormones, antibodies or vaccines made from this process, the proteins must be purified.
Until now, therapeutic protein purification technology has used protein-based affinity columns. However, they face financial and technological difficulties as the materials are costly, their storage or reuse is not efficient and their suitability and efficiency depends on the properties of each therapeutic protein.
The researchers succeeded in purifying various types of therapeutic proteins expressed in cells by harnessing the molecular affinity interaction using a synthetic host molecule cucurbit[7]uril (or CB[7] in short), which was first discovered by the same research group. The molecular affinity principle, a key element in the development of the purification technique, is dependent on the high-affinity and controllable host-guest interaction between CB[7] and guests such as adamantane.
The new purification technology has succeeded in purifying a monoclonal antibody drug, Herceptin (breast cancer treatment), as well as much smaller Interferon alpha (leukemia treatment) with high efficiency and high purity.
In particular, by applying small and stable synthetic molecules, the team succeeded in securing the manufacturability, sterilization, and recyclability of purified materials in a stable manner as well as increasing the purity and productivity of purified protein therapeutics. In addition, introducing adamantine (or AdA in short) to therapeutic proteins through genetic regulation and enzyme treatment can purify them regardless of their size or type.
This technique can be applied to most recombinant therapeutic proteins, including antibodies or fusion proteins that effectively prevent or treat fatal diseases such as viral infections or cancer, and is highly efficient and reusable. Furthermore, it is applicable to a wide variety of therapeutic proteins used in the development of vaccines or treatments which will speed up their production.
The research was conducted with the support from the Institute of Basic Science.
More information:
Jaeyeon An et al, Purification of protein therapeutics via high-affinity supramolecular host–guest interactions, Nature Biomedical Engineering (2020). DOI: 10.1038/s41551-020-0589-7
Provided by
Pohang University of Science & Technology (POSTECH)
New affinity purification technique for therapeutic proteins (2020, July 28)
retrieved 28 July 2020
from https://phys.org/news/2020-07-affinity-purification-technique-therapeutic-proteins.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com




