#Highly stable amyloid protein aggregates may help plant seeds last longer
“#Highly stable amyloid protein aggregates may help plant seeds last longer”
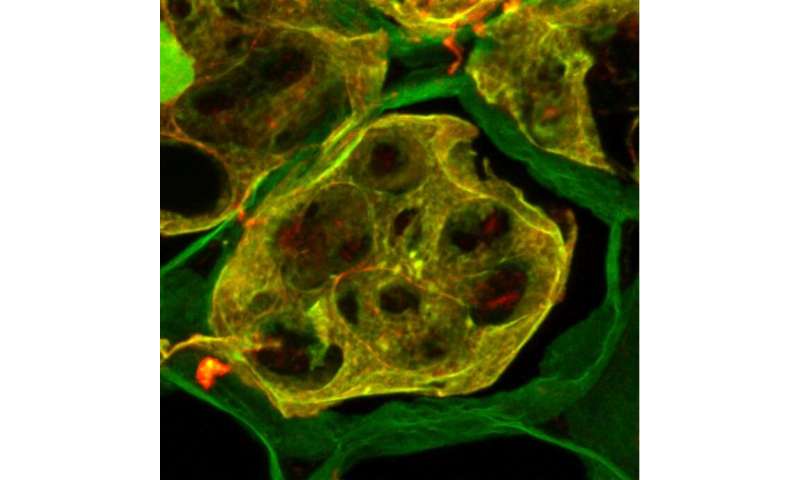
Highly stable polymeric “amyloid” proteins, best known for their role in Alzheimer’s disease, have been mostly studied in animals. But a new study on the garden pea publishing July 23, 2020 in the open-access journal PLOS Biology, by Anton Nizhnikov of All-Russia Research Institute for Agricultural Microbiology (ARRIAM) and colleagues, shows that they also occur in plants, and they may be an important adaptation for prolonging seed viability.
Amyloid is a type of protein conformation in which adjacent sheets of amino acids are bound together to form aggregates that are highly resistant to degradation. In animals, amyloids play roles in hormone storage and long-term memory formation, among other activities, but are best known from Alzheimer’s disease, which is characterized by the formation of plaques of amyloid aggregates in the brain.
Direct evidence that plants form amyloids has been limited, but recent biochemical experiments have hinted at it, and a recent bioinformatic study led by Nizhnikov turned up a group of seed storage proteins with amino acid sequences that suggested they might be able to form amyloids.
In the new study, working in the garden pea, the researchers extracted the seed storage proteins, including one called vicilin, and analyzed both the full-length protein and two its domains called cupins that are rich in predicted amyloidogenic regions. When the genes were engineered into bacteria, all three proteins formed amyloid fibrils resistant to strong detergents; they also bound amyloid-specific dyes, and displayed unique spectral properties, all indicative of bona fide amyloid structure.
In vivo, in the pea seed, the authors used an amyloid-specific dye and an antibody to vicilin to show that the two co-localized—where there was vicilin, there was amyloid-specific dye. Vicilin amyloid aggregates built up in the storage vacuoles during seed maturation, and then rapidly disassembled during germination, suggesting their role is as a nutrient reservoir. They also found that vicilin amyloids survived intact in canned peas, resisted treatment with protein-digesting gastrointestinal enzymes, and were toxic to yeast and mammalian cells.
“Amyloids are highly stable protein structures that resist different treatments and can, in several cases, persist in the external environment for decades,” Nizhnikov said. “Amyloid formation seems to be reasonable as evolutionary adaptation to provide for the long-term survival of plant seeds.”
More information:
Kirill S. Antonets et al, Accumulation of storage proteins in plant seeds is mediated by amyloid formation, PLOS Biology (2020). DOI: 10.1371/journal.pbio.3000564
Highly stable amyloid protein aggregates may help plant seeds last longer (2020, July 23)
retrieved 23 July 2020
from https://phys.org/news/2020-07-highly-stable-amyloid-protein-aggregates.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com


