#Cheap alloy rivals expensive platinum to boost fuel cells

“#Cheap alloy rivals expensive platinum to boost fuel cells”
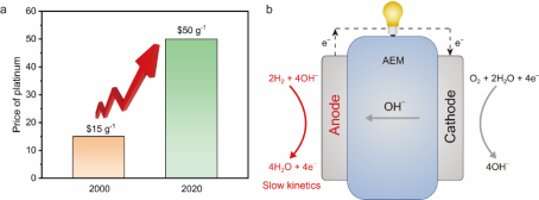
As the cleanest renewable energy, hydrogen energy has attracted special attention in recent research. Yet the commercialization of traditional proton exchange membrane fuel cells (PEMFCs), which consume hydrogen and produce electricity, is seriously restricted due to the chemical reaction of PEMFCs cathode largely reliying on expensive platinum-based catalysts.
A solution is to change the acidic electrolyte of PEMFCs to alkaline. Such fuel cells are called anion exchange membrane fuel cells (AEMFCs), and they allow for the use of cheaper metal elements like Co, Ni or Mn to design electrocatalysts.
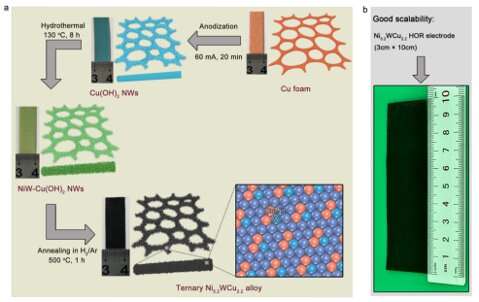
The research team led by Prof. Gao Minrui from University of Science and Technology of China (USTC) followed this solution and developed a practical and scalable way to manufacture a novel Ni-W-Cu alloy, Ni5.2WCu2.2, as the cathode for AEMFCs. The result was published on Nature Communications.
The team first grew Cu(OH)2 nanowires from a three-dimensional foam copper skeleton by anodic oxidation. The obtained nanowires were then immersed in a solution containing Ni and W elements. After hydrothermal synthesis and annealing, the Ni-W-Cu alloy was produced.
The ternary Ni5.2WCu2.2 alloy can catalyze the oxidation of hydrogen in alkaline medium 4.31 times more efficient than the benchmark platinum/carbon anode.
It has an oxidation potential as high as 0.3V in comparison with the reversible hydrogen electrode and can maintain high activity for up to 20h under such overpotential, proceeding anodes based on non-platinum-group metals.
The alloy catalyst also showed excellent resistance to CO poisoning, and maintained high activity in 20000 ppm CO/H2 mixed atmosphere.
Analysis showed that the projected density of states of Ni5.2WCu2.2 alloy lies in the lowest Fermi level, which indicates that the alloy has the optimal hydrogen binding energy. The multiple-element alloying effect renders the Ni-based alloy a high activity catalyst and offers oxidation resistance.
This work sheds light on further exploration of multiple-element alloys composed of cheap metals, thereby aiding the development of more efficient hydrogen oxidation catalysts for AEMFC anodes.
AI searched for single-atom-alloy catalysts, found 200 promising candidates
Shuai Qin et al, Ternary nickel–tungsten–copper alloy rivals platinum for catalyzing alkaline hydrogen oxidation, Nature Communications (2021). DOI: 10.1038/s41467-021-22996-2
Provided by
University of Science and Technology of China
Citation:
Cheap alloy rivals expensive platinum to boost fuel cells (2021, May 28)
retrieved 29 May 2021
from https://phys.org/news/2021-05-cheap-alloy-rivals-expensive-platinum.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you liked the article, do not forget to share it with your friends. Follow us on Google News too, click on the star and choose us from your favorites.
For forums sites go to Forum.BuradaBiliyorum.Com
If you want to read more Like this articles, you can visit our Science category.



