#An electrocatalyst for oxygen evolution reaction in water splitting
“#An electrocatalyst for oxygen evolution reaction in water splitting”
The transition from fossil fuels to renewable energy sources strongly depends on an availability of effective systems for energy conversion and storage. Considering hydrogen as a carrier molecule, proton exchange membrane electrolysis offers numerous advantages, like operation at high current densities, low gas crossover, compact system design etc. However, its wide implementation is hindered by slow kinetics of oxygen evolution reaction (OER), enhancement of which requires the application of low-abundant and expensive Ir-based electrocatalysts.
Looking for rational design of new types of OER electrocatalysts and addressing fundamental questions about the key reactions in energy conversion, the inter-institutional MPG-consortium MAXNET Energy integrated scientists from different institutions in Germany and abroad. As a result of close and fruitful collaboration within this framework, the scientists from Chemical Metal Science department at MPI CPfS together with experts from Fritz Haber Institute in Berlin and MPI CEC in Muelheim an der Ruhr, developed a new concept for producing multifunctionality in electrocatalysis and successfully illustrated it with an example of intermetallic compound Al2Pt as precursor for OER electrocatalyst material.
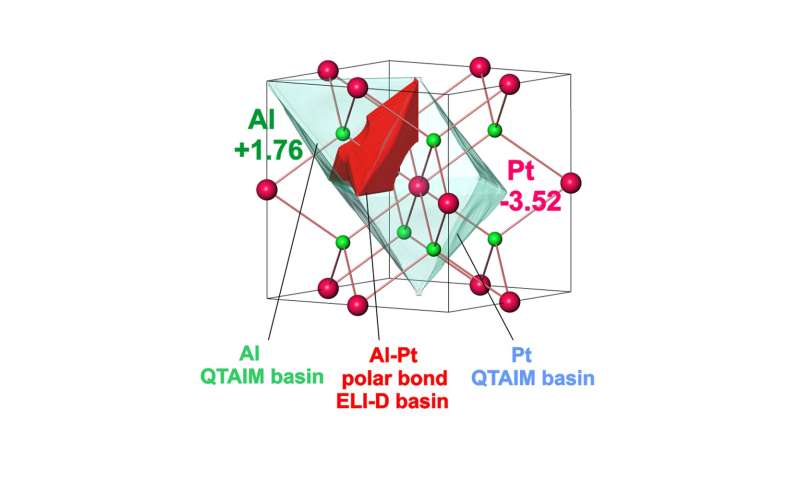
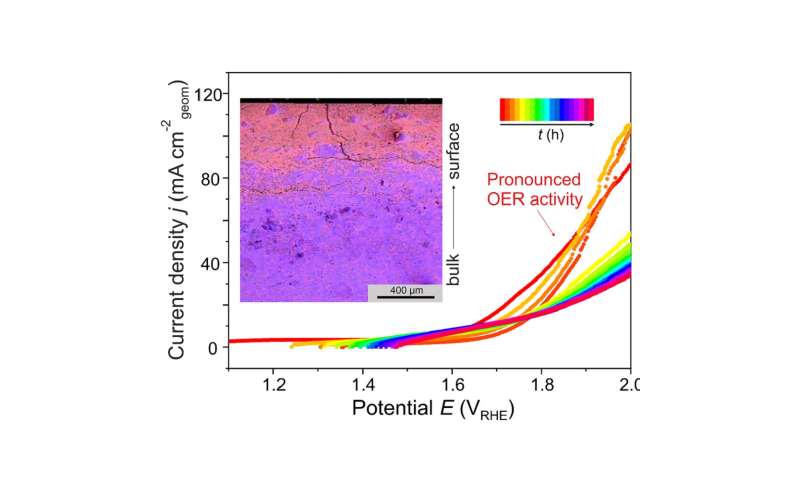
The intermetallic compound Al2Pt (anti-CaF2 type of crystal structure) combines two characteristics important for electrocatalytic performance: (i) reduced density of states at the Fermi level of Pt, and (ii) pronounced charge transfer from aluminum towards platinum, leading to strongly polar chemical bonding in this compound. These features provide inherent OER activity and increasing stability against complete oxidation under harsh oxidative conditions of OER. Upon OER conditions, Al2Pt undergoes restructuring in the near-surface region as a result of the self-controlled dissolution of aluminum. The roughness and porosity of in situ-formed near-surface microstructure allow to compensate the specific activity loss. Even after exceptionally long stability experiment (19 days) at high current densities (90 mA cm-2) the bulk material retains its structural and compositional integrity. Extending the choice of synthesis techniques, e.g. thin films growth, and exploring the variety of intermetallic compounds draw the main guidelines for future development of the proposed strategy.
The research at the Max Planck Institute for Chemical Physics of Solids (MPI CPfS) in Dresden aims to discover and understand new materials with unusual properties.
In close cooperation, chemists and physicists (including chemists working on synthesis, experimentalists and theoreticians) use the most modern tools and methods to examine how the chemical composition and arrangement of atoms, as well as external forces, affect the magnetic, electronic and chemical properties of the compounds.
New quantum materials, physical phenomena and materials for energy conversion are the result of this interdisciplinary collaboration.
More information:
Iryna Antonyshyn et al, Al2Pt for Oxygen Evolution in Water Splitting: a Strategy for Creating Multi‐functionality in Electrocatalysis, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202005445
An electrocatalyst for oxygen evolution reaction in water splitting (2020, June 26)
retrieved 26 June 2020
from https://phys.org/news/2020-06-electrocatalyst-oxygen-evolution-reaction.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
If you want to read more Like this articles, you can visit our Science category.
if you want to watch Movies or Tv Shows go to Dizi.BuradaBiliyorum.Com for forums sites go to Forum.BuradaBiliyorum.Com



